What processes does the alcohol go through before it can be classified as high percentage in the end? Our interview partner Kur Sartorius, initiator and leader of the “Schwäbisches Schnapsmuseums Bönnigheim” (Swabian Hard Liquor Museum in Bönnigheim) shares his expert knowledge.
BrüggemannAlcohol: What exactly is a Still?
Kurt Sartorius: Let’s first have a look at a distillation itself. This process represents a physical separation of two liquids. Water boils at 100 °C and alcohol at 78 °C. Consequently, when the liquid is heated, the alcohol evaporates first. The prerequisite for such a process is the use of a lockable vessel with an opening. This is where the pot still comes in. In it the mash is heated and brought to boiling. The alcohol-containing vapors are conducted to the cooler via the helmet and spirit pipe. There the vapors cool down and condense.
Only the cooler went through a series of developments: air coolers, tube coolers, plate coolers or rod coolers are a few development steps. In Baden-Württemberg, small distilleries use vessels with a capacity of up to 150 liters. Larger Stills can be found in Scotland, for example. They hold 5,000 liters.
A distillation column is fundamentally different. There the mash is pumped in at the top and runs down through various intensifying floors. Steam is introduced from below, which de-spirits the mash.
BrüggemannAlcohol: With which substance is a burning process started?
Kurt Sartorius: The mash is the starting material. It is an alcohol-containing liquid such as wine, fruit must or mashed fruit. Alternatively, the starting material can also be obtained from grain or seed which requires an intermediate step. This is because before distilling, the corn starch from the grain or seed has to be converted into fructose. The fructose is then fermented with yeast bacteria and thereby converted into alcohol.
Regardless of which mash is used as the starting material, it only contains around 7 - 8% alcohol by volume. After the first distillation, the alcohol content increases to 20% by volume. Within a further cycle, the liquid, now drinking brandy, reaches 40 - 50 % by volume.
BrüggemannAlcohol: How is high-proof alcohol made?
Kurt Sartorius: Each subsequent distillation process increases the alcohol content. That takes time and energy. Hence, distillation in the 19th century was very labor intensive. Thanks to new processes, it was finally possible to achieve a high alcohol content within one burning. At that time the use of amplification devices for the burning process began. Related constructions existed as early as the 16th century, but it was only with the invention of the Pistorius basin in 1817 that high-proof alcohol could be produced.
Using the Pistorius basin, water cools the alcohol-containing vapors in a lens-shaped container. Water condenses at 100 °C, alcohol at 78 °C. Therefore, water condenses first and the alcohol remains in vapor form. In further Pistorius basins, the alcohol is fortified to around 70% by volume in one burning only. The steam is then passed through a cooling device, where it condenses and an aqueous alcohol solution is ultimately created.
The amplifier devices that have been developed accelerate this enormously. The so-called bubble-tray is the most famous amplifier. Steam is passed through a bell to achieve the same effect: The water condenses and the alcohol remains in vapor form or evaporates again. By connecting several bubble-trays in series a higher alcohol content can be achieved. Today sieve trays can also be used in the same manner. At least 20 large bubble-trays are connected in series in powerful columns. Brüggemann, for example, uses this process and produces alcohol with 96% alcohol by volume.

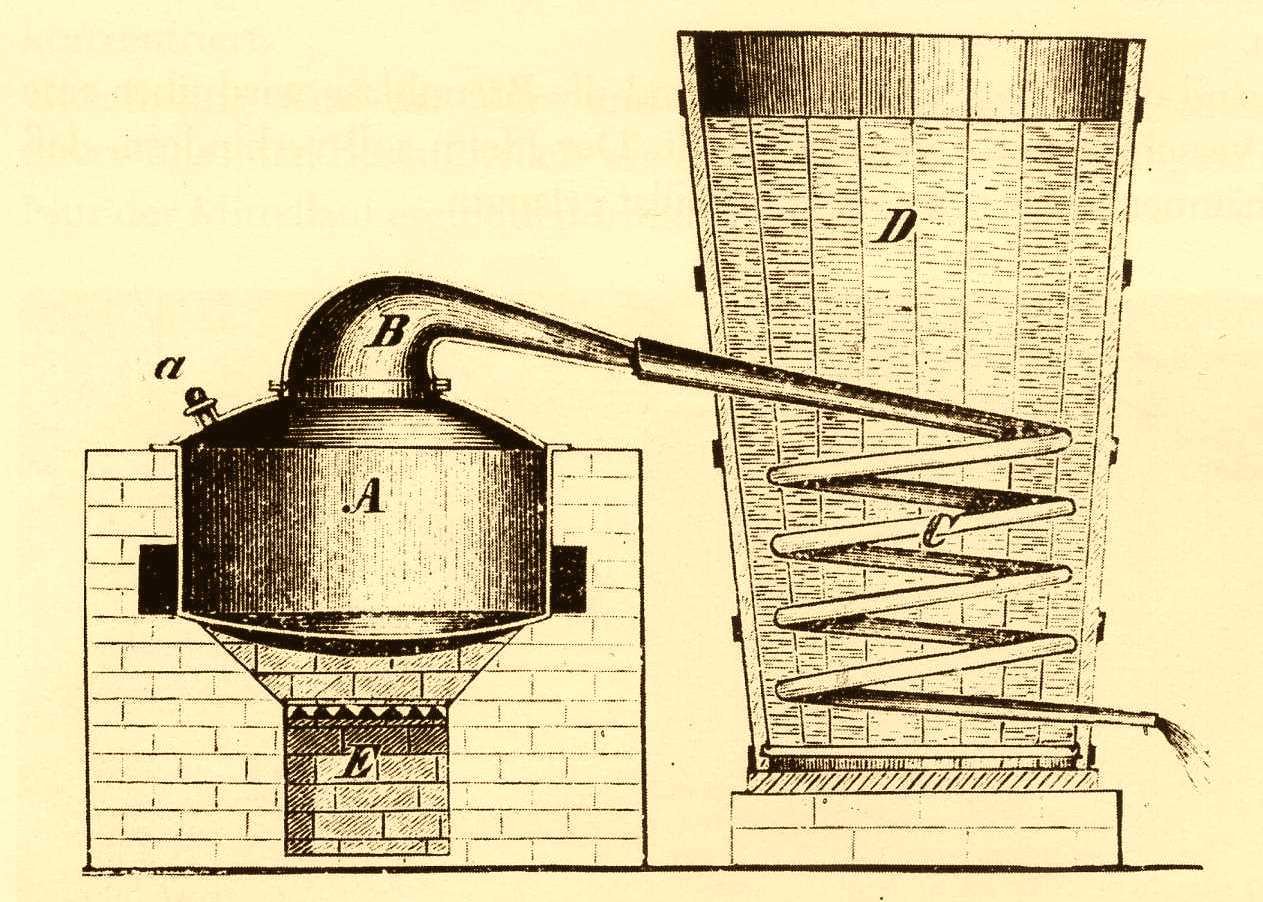
Picture Credits: left pricture: Brennblase, Helm und Geistrohr aus Kupfer (c) Schwäbisches Schnapsmuseum, right picture: Behrend Destillationsgerät mit Schlangenkühler, Kurzgefasste Anleitung zum praktischen Brennereibetrieb, 1885 (c) Schwäbisches Schnapsmuseum
BrüggemannAlcohol: What are the advantages and disadvantages of the still?
Kurt Sartorius: The advantage of the pot still lies in the resulting quality of the end product. Especially with fruit brandy, the aromas should be concentrated in the alcohol. This works better in the still than in the column. The helmet also has the advantage that it collapses the foam of the mash in a way that it does not get into the cooler and thus into the distillate. This would affect the quality and palatability of the product. For this reason, grain or potato mash is primarily distilled in the column.
BrüggemannAlcohol: Why is copper preferred for burning systems?
Kurt Sartorius: Copper is easy to work with or to bring into the required shape. The material proves to be a good heat conductor and very corrosion-resistant. Still, the main reason for choosing copper is because of its ability to bind sulfur. Such compounds are in the mash and without the chemical effect of copper, these compounds would pass into the distillate and affect its taste and quality.
If there is a person being entitled to call himself an expert of the history of alcohol, it definitely would be Kurt Sartorius, initiator und leader of the “Schwäbisches Schnapsmuseum Bönnigheim”. 44 years ago, he completed his first spirit distillery and henceforth intensively dealt with the history of alcohol. As a result, the distillery was to be turned into a local museum. Based on the recommendation by the state museum in 1985, the initial idea switched into the planning of creating a special museum. The already existing huge diversity of regional wine museums forced Mr. Sartorius to open up a schnapps museum (opening in 1993). Today Kurt Sartorius has Germany's largest alcohol history museum collection. In particular, distillation techniques, the development of alcohol history and illicit distillery are among his areas of expertise. In 2020 the “Schwäbische Schnapsmuseum Bönnigheim” was nominated for the German Berlin Spirit Awards of Tradition.